Cerebrospinal Fluid Stabilisation with TransFix® EDTA
TransFix Prevents Cellular Degradation in Cerebrospinal Fluid (CSF)
Cerebrospinal fluid (CSF or liquor) is a colourless bodily fluid found in the brain and spine which acts as a cushion or buffer from the brain’s cortex. CSF is obtained from the body via lumbar puncture; the back is anaesthetised and a needle inserted between the 3rd and 4th lumbar vertebrae. CSF is then collected under its own pressure.
Analysis of white blood cells in CSF is critical in the diagnosis of many infectious and inflammatory neurological disorders. As well as central nervous system involvement with lymphoproliferative disorders.
Flow cytometric analysis can provide absolute cell counts and detect rare events with high sensitivity and specificity, however cells within CSF samples are typically low in number, sparse in concentration and degrade rapidly ex vivo. This results in the necessity to promptly test CSF specimens to prevent false negative results (1)(2).
Features of TransFix
TransFix is a solution that stabilises leucocytes and leucocytic antigens for up to 14 days in multiple sample types, preserving the absolute cell counts and cellular markers. This allows for the differentiation of leucocyte sub-populations and provides an extended time frame for analytical testing.
TransFix has been shown to stabilise malignant haematological cells in cerebrospinal fluid, making it possible to determine leucocyte subsets in CSF via flow cytometric analysis 72 hours after lumbar puncture. TransFix has therefore been has been recommended in guidelines published in the British Journal of Haematology (see case study 1).
• TransFix/EDTA CSF Sample Storage Tubes are specifically designed for the stabilisation of cells within CSF specimens.
• Higher lymphocyte yield after 30 minutes compared to untreated samples (see case study 2).
• The cellular component of CSF is stabilised for at least 72 hours allowing for determination of cell sub-populations and follow up analysis (see case study 3).
• Light scatter and key antigen expression patterns are comparable to fresh samples (see case study 3 and case study 4).
• Markers stabilised include Markers stabilised include CD10, CD19, CD34 (see case study 3 and case study 4), CD45, HLA-DR, and CD117 (see case study 3).
Benefits of Cerebrospinal Fluid Stabilisation
• More time in which to conduct testing
• Allows for further investigation after initial cytomorphological analysis has identified malignant cells, using an aliquot of the same sample.
• Allows determination of leucocytic cell populations via flow cytometric analysis, up to 3 days after collection.
• Reduced cell lysis during transportation of samples from the collection site to the laboratory.
• Reduced need for repeat lumbar puncture
• Reduced costs
• Reduced trauma for child patients and parents
• Reduced time spent re-sampling
• Standardise Results:
• The ability to batch samples for transportation and testing
• Reduces lab variability
• Beneficial for clinical research studies: Haematologica 2014;99(7) p1228-1235
TransFix Stabilisation of Malignant Haematological Cells in Cerebrospinal Fluid
Leukemic infiltration of the CNS is a sign that leucocytes have spread to the brain and spinal cord, and is often seen in diseases such as childhood acute lymphocytic leukemia. Flow cytometry is proving to be useful detecting malignant cell populations in CSF, however low cell counts and rapid decline of cells after lumbar puncture can pose technical challenges for flow cytometric analysis.
TransFix has been used for the stabilisation of malignant haematological cells found in CSF. TransFix/EDTA CSF Sample Storage Tubes contain TransFix that has been optimised for CSF stabilisation and have been recommended in guidelines for CSF sample storage, published in the British Journal of Haematology (see case study 1).
TransFix has also been shown to reduce cellular lysis after 30 minutes compared to fresh CSF (see case study 2) and to stabilise key CSF leucocyte markers for up to 72 hours (see case study 3). The stabilisation process preserves light scatter and key antigen expression patterns allowing analysis of diagnostic and follow up CSF specimens for patients with CNS infiltration (see case study 3 and case study 4).
Importance of Flow Cytometric Analysis of Haematological Malignancies in CSF
Invasion of leucocytes into cerebrospinal fluid has grave prognostic significance and requires important therapeutic decisions including the administration of intrathecal chemotherapy. Leptomeningeal localisation is conventionally diagnosed by cytomorphological analysis through identification of malignant lymphocytes in CSF, however this technique has a relatively high rate of false-negative results in up to 60% of cases. Recent reports suggest that multiparameter flow cytometric assessment of CSF samples could improve the efficiency of detection of CNS involvement, due to its high specificity and greater sensitivity (1).
Case Study 1: Guidelines on the use of multicolour flow cytometry in the diagnosis of haematological neoplasms.
Johansson U, Bloxham D, Couzens S, Jesson J, Morilla R, Erber W, Macey M and British Committee for Standards in Haematology. British Journal of Haematology (2014) 165: 455-488
These guidelines have been developed by UK-based clinical flow cytometry (FC) experts along with clinical representatives to take into account advances in the state of the art of clinical flow cytometry since 2002. Their work was revised by the Haemato-oncology Task Force of the British Committee for Standards in Haematology (BCSH) before review by other UK-based Haematologists and FC experts, the BCSH and the British Society for Haematology committee. This document covers a varied range of topics, from selection and setup of flow cytometers through data analysis to training considerations for staff working in the clinical FC laboratory.
The guidelines strongly recommend sample age and quality is assessed prior to analysis and in the case of cerebrospinal fluid (CSF), it is recommended that unless immediate analysis is possible then samples can be stored in TransFix/EDTA CSF Sample Storage Tubes for up to 72 hours before analysis*.
*TransFix/EDTA CSF Sample Storage Tubes are currently a Research Use Only product and Cytomark recommends that internal validation of the product must be completed by the end user before considering use of the products in a clinical setting.
Case Study 2: Use of TransFix Cerebrospinal Fluid (CSF) Storage Tubes Prevents Cellular Loss and Enhances Flow Cytometric Detection of Malignant Hematological Cells After 18 Hours of Storage
In patients with suspected or proven leptomeningeal localised haematological malignancies (LHM) in CSF, rapid assessment is required as cell numbers decline quickly after lumbar puncture. The study aim was to examine leucocyte numbers and detection of LHM by flow cytometry in 99 CSF samples from patients with suspected or proven LHM after 30 minutes and 18 hours of storage in i) TransFix/EDTA CSF Sample Storage Tubes ii) serum-containing medium and iii) no stabilising agent (untreated). It was found that leucocyte numbers in TransFix stabilised CSF were higher than in the corresponding untreated samples at both time points. After 18 hours of storage at 4°C, the detection of LHM was enhanced in TransFix stabilised CSF. For all discordant paired observations, the level of suspicion of a patient having LHM was higher for the TransFix stabilised samples than for the untreated samples. The authors concluded that the use of TransFix/EDTA CSF Sample Storage Tubes prevents cellular loss and enhances flow cytometric detection of LHM after 18 hours of storage.
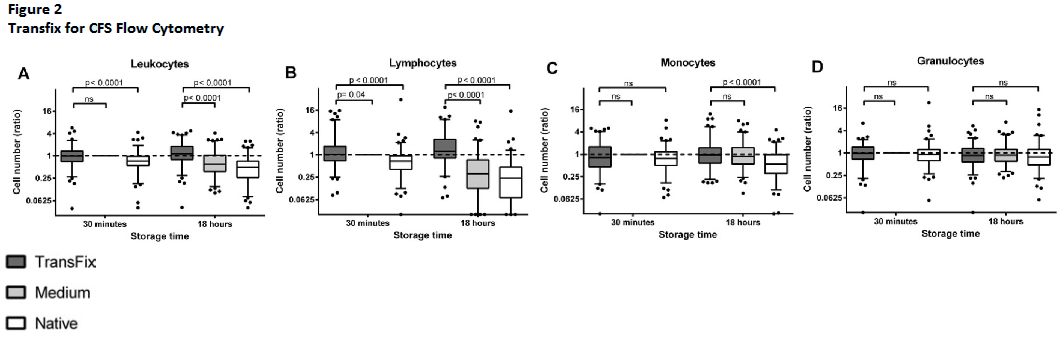
FIG. 2. Relative numbers of leucocytes (A) and their subsets (B–D) after 30 minutes and 18 hours of storage in CSF with TransFix®, CSF with serum-containing medium, and native CSF. Relative numbers were calculated by dividing the absolute numbers (in cells/mL) by those in CSF with serum-containing medium at 30 minutes. A reference line is drawn at a relative cell number of 1, to indicate the cell number in serum-containing medium at 30 minutes. Boxes represent medians and quartiles, whiskers 5th and 95th percentiles. P values were calculated with the Wilcoxon signed rank test. ns, not significant.
Cytometry Part B (Clinical Cytometry) 000B:00–00 (2013)
*TransFix/EDTA CSF Sample Storage Tubes are currently a Research Use Only product and Cytomark recommends that internal validation of the product must be completed by the end user before considering use of the products in a clinical setting.
Case Study 3: Infiltration of CNS by acute leukaemia: Analysis of fresh and stabilised CSF
A research study to determine whether acute leukaemia blasts present in CSF can be successfully stabilised by TransFix has concluded that Transfix preserved light scatter and key antigen expression patterns allowing analysis of diagnostic and follow up CSF specimens for patients with CNS infiltration.
Full evaluation of the antibody panels to be used on transfixed samples is also required since some antigens are expressed dimly.
Figure 1: Transfix-induced light scatter changes do not impair cell identification
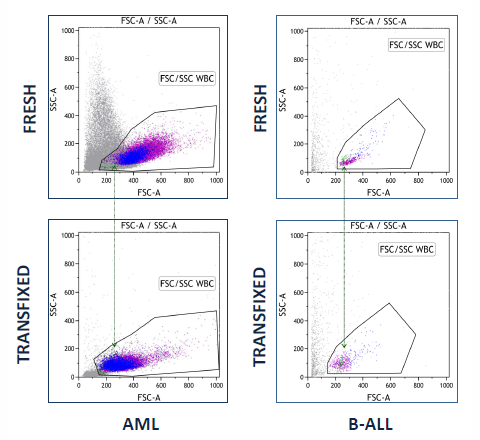
Figure 1. Transfix-treated cells had reduced FSC compared to fresh cells, for all cell populations and samples examined (n=23). All cases of B-Acute Lymphocytic Leukaemia (B-ALL) (n=4) showed an increased SSC signal after transfix treatment. No sample or cell population were difficult to gate or recognise due to transfix induced light scatter changes.
Figure 2: TransFix maintains key B-ALL antigen expression
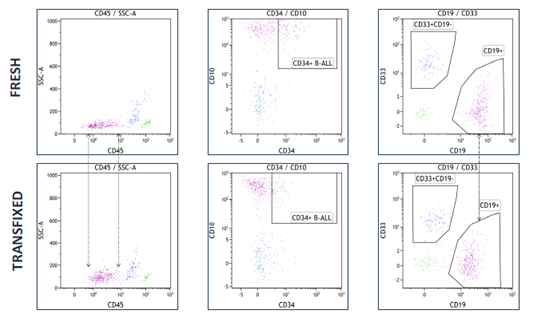
Figure 2. Example of a post-treatment, follow-up CSF sample from a B-ALL patient. CD19, CD34 and CD10 have reduced signal on transfix treated cells; however this did not hamper cell recognition or gating.
Figure 3: TransFix maintains key myeloid antigen expression
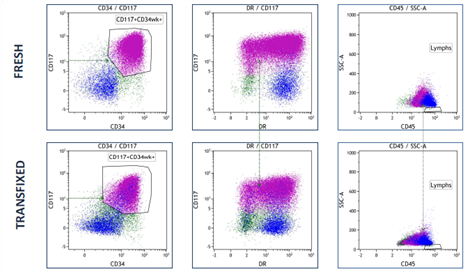
Figure 3. Example of a presentation CSF sample from a patient with central nervous system (CNS) infiltration of acute myeloid leukaemia. Expression of CD45, HLA-DR, CD34 and CD117 was maintained to allow cell identification and gating.
TransFix/EDTA CSF Sample Storage Tubes are currently a Research Use Only product and Cytomark recommends that internal validation of the product must be completed by the end user before considering use of the products in a clinical setting.
*In collaboration with Haematology Dept, Bristol Royal Infirmary, University Hospitals Bristol NHS Foundation Trust, UK.
Case Study 4: CSF Screening for Suspected CNS localised leukemia or lymphoma
Evaluation studies into the use of TransFix/EDTA for stabilising CSF samples from patients with suspected CNS localised leukemia or lymphoma are being conducted*. Results to date indicate the following:
• Lymphocyte cell recovery of most subsets observed in TransFix/EDTA treated CSF samples matched or exceeded that of the fresh samples (Graph 1).
• Antigen expression profiles in TransFix/EDTA treated CSF are maintained for 3-7 days and are comparable to equivalent fresh, untreated CSF samples (Figure 1-4).
• The diagnostic conclusion would have remained the same for all patients using TransFix/EDTA treated samples compared to fresh (Figures 1-4).
• Results suggested that TransFix/EDTA can be used successfully to stabilise CSF samples for screening purposes.
Graph 1: Lymphocyte cell recovery within TransFix treated & untreated CSF samples
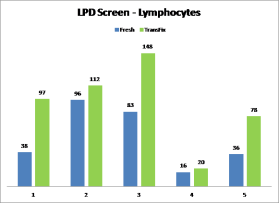
Graph 1. The number of lymphocyte events recorded in fresh (blue) and TransFix treated (green)CSF from 5 patients suspected of Lymphoproliferative Disorders (LPD)
Figures 1 – 4 : B-blast cells in a TransFix treated & untreated CSF sample
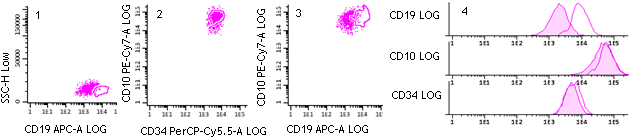
Figure 1-4: Identification of B-blast cells in a patient with B-ALL. Pink populations denote B-blasts in the TransFix stabilised CSF sample analysed after 7 days. The ringed (clear) populations denote the same cells in the fresh sample. Fluorescence intensity was reduced for some mAbs in TransFix samples, but this did not impair gate settings or change the diagnostic conclusion.
*TransFix/EDTA CSF Sample Storage Tubes are currently a Research Use Only product and Cytomark recommends that internal validation of the product must be completed by the end user before considering use of the products in a clinical setting.
*In collaboration with Haematology Oncology Diagnostics at the Bristol Royal Infirmary.
Product Information
TransFix/EDTA CSF Sample Storage Tubes consist of 5ml screw cap tubes containing 0.2 ml of Transfix/EDTA, which can be used to stabilise up to 5ml of CSF. These tubes are available in packs of 10, 25 and 50. TransFix/EDTA CSF Sample Storage Tubes must be stored at 2-8°C.
Sample sizes are available for evaluation.
| Product Code | Description | Quantity |
|---|---|---|
| TF-CSF-5-2 | CSF Sample Storage tubes containing 0.2ml TransFix/EDTA | 2 x 5ml |
| TF-CSF-5-10 | CSF Sample Storage tubes containing 0.2ml TransFix/EDTA | 10 x 5ml |
| TF-CSF-5-25 | CSF Sample Storage tubes containing 0.2ml TransFix/EDTA | 25 x 5ml |
| TF-CSF-5-50 | CSF Sample Storage tubes containing 0.2ml TransFix/EDTA | 50 x 5ml |





